When the Fda Conducts an Inspection the Inspectors Will
An inspection is defined as the act by a regulatory authority of conducting an official review of documents facilities records and any other resources related to a clinical trial. Upon arrival the FDA investigator will present credentials and a Notice of Inspection FDA Form 482.

What Is The Best Way To Prepare For An Fda Inspection Redica
FDA inspections or audits are in-person site visits performed on c linical investigators s tudy sponsors or IRBs.

. Office of Regulatory Affairs ORA. To start the inspection off on the right foot a knowledgeable person from the facility such as the plant or production manager should be designated ahead of time. And 3 for-cause inspections which are conducted to investigate particular issues such as those arising.
Pre-approval inspection after a company submits an application to FDA to market a new product. 1 preapproval inspections which are conducted prior to when a drug is marketed in the US. Ensure the protection of human research subjects and data integrity.
FDA conducts several types of inspections to help protect consumers from unsafe products. The Food and Drug Administration FDA conducts inspections and assessments of regulated facilities to determine a firms compliance with applicable laws and regulations such as. For-cause inspections are conducted in response to information the agency considers a potential public health risk.
For example if an inspector visits a facility and issues an FDA Form 483 noting several deficiencies the FDDA will conduct a follow-up inspection to ensure the management addressed the concerns. Who conducts inspections for FDA. FDAs Office of Regulatory Affairs ORA is the lead office for all field activities including inspections and enforcement.
Types of FDA Inspections. During an inspection ORA investigators may. Food and Drug Administration.
FDA investigators in FDA District Offices. The FDA resumed conducting domestic surveillance inspections across all commodities yesterday February 7. The FDA resumed normal domestic inspections of drug facilities yesterday citing the declining rates of COVID-19 cases across the country.
7 the agency will resume conducting domestic surveillance inspections across all commodities given the decline in COVID-19 cases across the. 2 the FDA determined that beginning on Feb. In fact the number of on-site inspections of medical device manufacturers conducted between March 2020 and March 2021 totals fewer than 100 and most of those were done in the US in early March.
The overall goal of monitoring audits and inspection activities is to. Inspections conducted by States pre-approval inspections mammography facility inspections inspections waiting for a final enforcement action and. Office of Regulatory Affairs Field Operations.
29 that it was temporarily suspending many of its domestic and foreign inspections due to fast-spreading SARS-CoV-2 Omicron variant. A systematic and independent examination of trial-related activities and documents is an audit and the act of overseeing the progress of a clinical trial is monitoring. 2 surveillance inspections which are conducted after a drug is marketed in the US to evaluate continued GMP compliance.
The agency said it will continue to use a variety of. Food and Drug Administration FDA conducts inspections to protect the rights safety and welfare of research study participants verify the accuracy and reliability of study data and assess compliance with FDA regulations for the conduct of clinical trials. The investigator will present.
An investigators perspective FDA Inspections Summit Bethesda MD October 23 2014 Lori S. FDA conducts three main types of inspections. The FDA had announced on Dec.
That person should then greet the investigator accompany him or. House Appropriations Chair Rosa DeLauro D-Conn meanwhile has asked the Health and Human Services Office of the Inspector General to conduct an independent investigation. The FDA conducts three types of inspections.
Foreign surveillance inspections that have received country clearance and that are within. Preapproval inspections generally support New Drug Applications NDAs Premarket Approvals PMAs and licensing agreements. The FDA maintains its.
FDA may conduct an inspection of your operation for a variety of reasons such as a routinely scheduled investigation a survey or a response to a reported problem. When the FDA conducts an inspection the inspectors will. On August 23 through September 7 2021 the Food and Drug Administration FDA conducted a remote Foreign Supplier Verification Program FSVP inspection of Elis Manhattan Warehouse Inc.
This guidance is intended to provide information about FDA inspections of clinical investigators conducted under FDAs Bioresearch Monitoring BIMO Program. On August 20 2021 through September 3 2021 the Food and Drug Administration FDA conducted a Foreign Supplier Verification Program FSVP inspection of Agrosons LLC located at 735 Drake. According to ICH E6 GCP an Audit is defined as.
FDA is obligated to conduct these inspections and is facing growing pressure from the US Government Accountability Office GAO to resume inspections. Not all inspections are included in the database. FDA has determined that MDSAP audits do not meet the criteria for posting on the FDA Data Dashboard.

Audits Inspections In Clinical Research

Audits Inspections In Clinical Research

Shedding Light On An Fda Visit Ppt Download

Audit And Inspection In Clinical Trial Objective
What Happens During An Fda Inspection

Fda Seeks Risk Based Inspection Program Pharmaceutical
Nontransferable Obligations Why Sponsors Fail Inspections Florence

What To Expect During An Fda Inspection Emma International

Here Are The 4 Types Of Fda Inspections You Need To Understand

Audits Inspections In Clinical Research

Audits Inspections In Clinical Research

Audits Inspections In Clinical Research

Pdf Ready For Pharmacovigilance Inspection Usfda
.png?width=2400&name=5%20tips%20(4).png)
How To Prepare For An Fda Inspection Plus 5 Tips
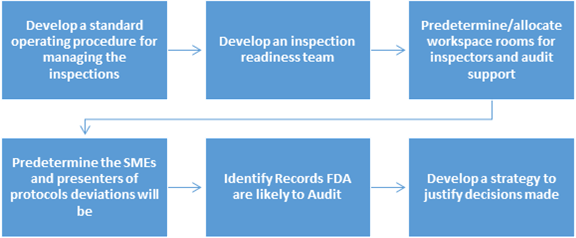
Fda Inspection Guide What To Do Before During And After The Fda Inspection

The Utility Of Remote Inspections During The Covid 19 Health Emergency And In The Postpandemic Setting Clinical Therapeutics

Audits Inspections In Clinical Research

Getting Through Inspections Seasoned Research Institution Details Successful Strategies 2021 09 24 Centerwatch

Observations From Gcp Sponsor Inspections Clinical Trials Arena
Comments
Post a Comment